Ferro Alloys
Nickel is a naturally occurring, lustrous, silvery-white metallic element. It is the fifth most common element on earth and occurs extensively in the earth's crust. However, most of the nickel is inaccessible in the core of the earth.
Nickel metal’s key characteristics are:
- High melting point, 1453 ºC
- Resists corrosion and oxidation
- Very ductile
- Alloys readily
- Magnetic at room temperature
- Can be deposited by electroplating
- Has catalytic properties
| Name: | NICKEL |
| Chemical Symbol: | Ni |
| Atomic Number: | 28 |
| Atomic Weight: | 58.71 |
| Melting Point: | 1453 ºC |
| Boiling Point: | 2730 ºC |
| Density: | 8.90 g/cm3 at 25 ºC |
| Curie Temperature: | 253 ºC |
Reflecting these characteristics, nickel is widely used in over 300,000 products for consumer, industrial, military, transport, aerospace, marine and architectural applications. The biggest use is in alloying - particularly with chromium and other metals to produce stainless and heat-resisting steels. These are used for pots and pans, kitchen sinks etc, as well in buildings, food processing equipment, medical equipment and chemical plants.
About 65% of the nickel which is produced is used to manufacture stainless steels. Another 20% is used in other steel and non-ferrous alloys - often for highly specialized industrial, aerospace and military applications. About 9% is used in plating and 6% in other uses, including coins, electronics, and in batteries for portable equipment and hybrid cars. In many of these applications there is no substitute for nickel without reducing performance or increasing cost.
Nickel is essential for healthy plant life. As a result, it is found naturally in most vegetables, fruits and nuts, and in the food products derived from them, for example – chocolate and wine.
How Nickel is Recycled
Metals are elements, part of the periodic table. They cannot be created artificially nor destroyed. The processes of mining and, depending on the nature of the ore, smelting, leaching and/or refining are used to take concentrations in nature (“deposits”) and further concentrate them to useful levels of purity. The use phase of metals, including alloys, doesn’t impact on that level of purity although there are losses due to wear, erosion or corrosion. At the end of the first use phase, however, the metals and alloys can be recovered and recycled.
Depending on the economics, of which energy and chemical usage are prime determinants, the metals and alloys can be returned to their original state or a different but still valuable state. Examples involving nickel would be nickel-containing stainless steel scrap being turned into new stainless steel (same to same) or nickel from recycled batteries going into nickel-containing stainless steel (former use to new form).
Note: "scrap" refers to material that has been used and is available for recovery. A common synonym also used is "secondary material”. This is in contrast to "primary" or "virgin" material that comes from mine production.
The following sections show that while nickel is highly recycled, it is not a single path but many separate alloy recycling loops. Very little nickel is recycled as nickel. Methodologies that quantify nickel recycling (indices, recycling rates, etc.), need to define the nickel cycle broadly enough to embrace all these "alloy loops".
Recycling Nickel :
Nickel is rarely used by itself but is commonly mixed with other metals to produce alloys. This is very different from, for example, copper. There are thousands of different alloys containing nickel - each developed to offer a particular combination of technical properties (corrosion resistance, mechanical properties and service life) relevant to particular conditions of use.
The nickel content of these alloys varies widely from, as examples, 1-3% for special engineering steels, 8-14% for stainless steels, 15-40% for special engineering alloys, 40-90% nickel for special alloys for the aerospace and electronic industries.
The nickel content of these alloys varies widely from, as examples, 1-3% for special engineering steels, 8-14% for stainless steels, 15-40% for special engineering alloys, 40-90% nickel for special alloys for the aerospace and electronic industries.
It is usual practice for special alloys to be recycled as the same special alloy wherever possible: the stringent specifications and the cost of achieving them in the first place can justify them having their own closed loops: production of a specific, its use phase and then collecting and recycling that alloy material to produce “new” material that matches the original specification. The motivation is economic. If the identity of the alloy can be maintained from fabrication to end-of-life of the component, the alloy producer can use that scrap alloy to make new alloy components. This is economically and environmentally efficient as it allows the producer to achieve high quality product specifications without incurring extra refining or qualification costs.
In practice it is not always possible to maintain and segregate products and scrap into specific alloys. Alloys and products get mixed. The nickel recycling industry has various ways of handling mixed nickel-containing scrap material in order to optimize the retained value of the scrap. One common technique is to melt the mixture in order to produce "remelt" ingot of a known composition for subsequent resale.
A variant of this is to adjust the composition of the remelted scrap by adding controlled amounts of primary metals in order to produce ingot to a required specification for resale. A further technique is to "blend" recycle material from different sources to produce a mixture which, when subsequently melted by the purchaser, will produce a melt with a specified composition. This blending process is of increasing importance in stainless steel production.
Recycling Nickel :
The main first-use industry for nickel is stainless steel.
Nickel-containing stainless steels (there are other kinds) commonly contain between 8 and 14% nickel, and account for approximately 60 percent of primary nickel use. For more on stainless steel, see "Recycling by First-Use Industry".
The modern refining processes used to produce stainless steel allow a wide range of raw materials to be used economically, of which scrap from stainless steel products is only one.
Sophisticated "blending" processes are used by specialist suppliers in order to provide quality-assured feed to stainless steel mills. These blending processes can utilise nickel-containing products from a very wide range of fabricating or end-of-life sources - including low-nickel steels; high nickel alloys; mixed turnings; end-of-life engineering assemblies; reject products from primary nickel producers; and re-melted ingot from processing nickel-containing slags, dusts, batteries, and spent plating fluids.
This "omnivorous" character of the stainless steel industry means the stainless steel industry puts a higher value on many of these products than does the industry which originally generates the products. Hence, many products become feed for the stainless steel loop rather than feed for the industry sector that originally produced the products.
Economics of Recycling
Non-ferrous metals are among the most intensively recycled materials in modern economic life. This situation is not the result of recent political encouragement or life style changes. It is the result of centuries of economic development. Several common features of non-ferrous metals have stimulated this development.
Non-ferrous metals are commonly found in the earth but are difficult to find in concentrated form (a deposit), and difficult to extract and refine. This means that non-ferrous metals produced from primary sources sell for relatively high prices.
"Primary" Nickel and "Secondary" Nickel
All the nickel available to society today originally came from a mine.
The first time it came from a mine and went through smelter and/or refinery processes to become a usable metal, it was called "primary" nickel. When it came around for a second or subsequent time in industrial processes it was and is called "secondary" nickel.
While all nickel was once "primary" nickel, it does not follow that all "primary" nickel is new nickel.
While nickel is amongst the world's most highly recycled substances, the vast majority is recycled by the stainless steel, steel, copper and brass industries - and by companies that supply those industries with material. But the "primary" nickel industry also takes in some "secondary" nickel, especially in its smelters.
In fact, there are some industrial wastes that are unattractive to the big recyclers of nickel. In such cases the omnivorous nature of nickel smelters may mean that a nickel-containing waste will be used that otherwise would go to landfill. Other times it is simple economics: which industry will pay the highest price for the nickel-containing material? Or which industry is closest to the material and thus has a smaller transport cost?
The "primary" nickel produced by the member companies of the Nickel Institute (roughly 75% of world annual production) contains approximately 2% nickel from "secondary" materials.
As the amount of nickel becoming available for recycling increases, the recycle content in "primary" nickel can be expected to increase modestly over time.
In most cases, however, it will be environmentally and economically more efficient for most nickel to be recycled as it is today: by the stainless steel industry.
Objectives
To this end, the metals industry supports the characterization and modeling of recycling of metal-containing products in a way that:
- Encourages good environmental practices;
- Aids assessment of the overall life cycle of products and understanding of materials;
- Supports the management of the life cycle of products and stewardship of materials;
- Is consistent with scientific knowledge and technical practices; and
- Reflects economic realities without creating market distortions that impede environmental objectives.
About Metal Recycling
Metals are highly recyclable and in fact a large percentage of metallic material is effectively recycled. Collected metal scrap is converted to new material of equal or similar quality through metallurgical processes, including remelting and refining. Some products require metal grades that demand minimal processing; other products may require more metallurgical and process controls to meet specifications
Facts
The following are relevant to metals recycling:
- Recycling of metals has environmental, economic and social value. Consequently, and for many years, metals from end-of-life products are widely recycled at high rates.
- Recycled metal is readily sold on the market. The constraint to greater levels of metal recycling is the availability of feedstock material.
- Metals are characterized by metallic bonding that provides distinct structures and properties. As this type of bonding is not affected by melting, metals can be, and are, recycled over and over again.
- Material grade is determined by conformity to established specifications. The origin of metal (whether primary or recycled) in a specific lot of material is driven by availability and economics.
- Metal may be lost during product use (e.g., via corrosion or wear), and some material may not be economically recoverable at end-of-life due to material dispersion or difficulties in separating components.
- Since there is growth in the demand for metals and since metal products often have a long service life, there is a limited supply of used metals available for recycling into new products. Primary metal production fills the gap between the availability of secondary material and total demand.
Where & Why Nickel is Used
Nickel-containing materials play a major role in our everyday lives – food preparation equipment, mobile phones, medical equipment, transport, buildings, power generation – the list is almost endless. They are selected because - compared with other materials - they offer better corrosion resistance, better toughness, better strength at high and low temperatures, and a range of special magnetic and electronic properties.
Most important are alloys of iron, nickel and chromium, of which stainless steels (frequently 8-12% nickel) are the largest volume. Nickel based alloys - like stainless steel but with higher nickel contents - are used for more demanding applications such as gas turbines and some chemical plants.
In addition, iron and nickel alloys are used in electronics and specialist engineering, while copper-nickel alloys are used for coinage and marine engineering.

- Spent Nickel Catalyst
- Nickel Concentrates
- Nickel Residues
- Nickel Bearing Scraps
- Spent Molybdenum Catalyst
- Copper / Manganese / Cobalt / Vanadium Spent Catalysts
- Turnings / Grindings
- Metal Sludges
Major Advantages of Installing the Plant in Haryana :
- Availability of ample Raw Material & Power in Surrounding area
- Processing of waste in the Home country and make the value added products
- Decrease in Imports as Nickel is the import substitute item
- Centralized location among Asian economies
- Availability of Skilled , Un-skilled & semi-Skilled Man power
- Contributes in Trade balance
Process & Technology :
The complete process being established is Eco-Friendly and Green Technology and there is Zero Waste discharge and no environmental hazard.
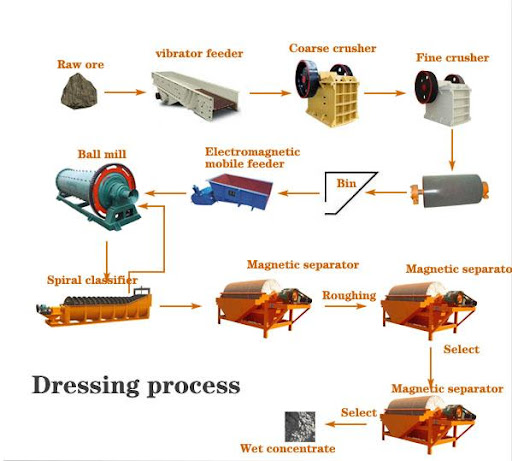

COMPILED EXTRACTION PROCESS
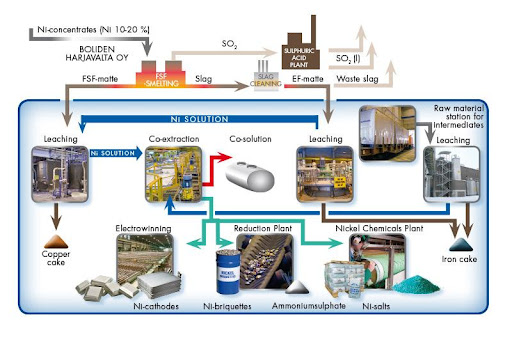
PYROMETALLURGY PROCESS OF FERRO-NICKEL AND OTHER ALLOYS
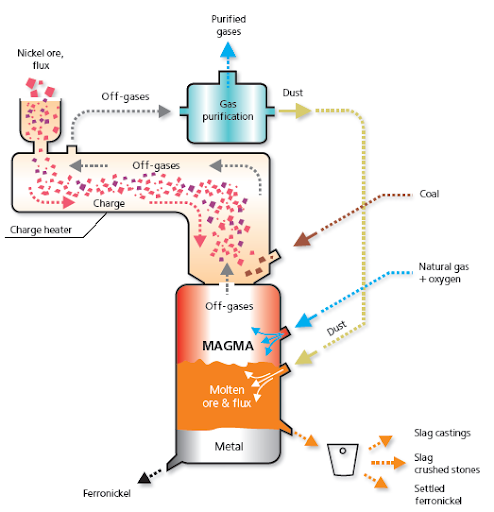
FERRO NICKEL
Nickel is a very important alloying metal which finds application in many cast irons and steels. The main reason for adding nickel in ferrous alloys is to promote an austenitic microstructure. Nickel generally increases ductility, toughness and corrosion resistance. There are two main categories of nickel products available for foundries, nickel metal, which is available in a variety of shapes and sizes, and ferronickel.
In general, foundrymen favor pure metals over ferroalloys because of their ease of use. However, producing ferroalloys rather than pure metals has some advantages over refining them to highly purified metals. In fact, the refining and concentration process is costly and it is not logical to remove iron while it is the main element in ferrous melting.
Nickel is an essential and widely used alloying element in high-temperature-resistant superalloys and heat-, oxidation-, and corrosion-resistant irons and steels. Nickel is well-known for its solid solution strengthening and promoting of high toughness, mainly at low temperatures. Besides its main application in stainless steelmaking nickel is also widely used in low-alloys steels and irons.
The addition of higher amounts of nickel promotes austenite stabilization at room temperature together with loss of ferromagnetism and increasing resistance to corrosion. Because of its little or no affinity to react with carbon, nickel has graphitization effects in ferrous alloys which are needed for the characteristic graphite microstructure of cast iron. For steels, this phenomenon needs to be stopped by keeping carbon in low percentage amounts or adding carbide forming elements like chromium. Furthermore, nickel inhibits the grain growth and increases the hardenability in ferrous alloys.
Nickel products for foundries
Ferronickel and Nickel Pig Iron (NPI) are two alternatives to nickel metal. NPI typically contains around 4 to 10 wt% nickel. Its high amount of carbon (>3 wt%), silicon (>3 wt%) and manganese (>1 wt%) mean that its application in foundries is highly restricted. In fact, the main consumers of this product are stainless steel mills in China, because of their capacity to reduce carbon and other undesired elements [5]. Ferronickel is a ferroalloy containing mainly iron and nickel. It is supplied in the shape of flowable granules with a typical particle size between 2 and 50 mm. Part of the production process is a refining step that reduces the amount of carbon, silicon, manganese, sulfur, and phosphorus to an acceptable level for foundries